Biogen and Eisai recently stopped two phase 3 trials of the Alzheimer’s treatment aducanumab after 36 months due to lack of efficacy. The 3,200 people in the trials and their families reacted with a sense of hopelessness and resign, particularly as there is no other treatment or clinical trial option for them. (STAT)
Profit motive and aversion to risk limit Alzheimer’s progress
Large pharmaceutical companies have been cutting back on their in-house Alzheimer’s research and development. The lack of progress in the fight against Alzheimer’s likely has more to do with the world of finance than the world of biology. Pharmaceutical companies are not able to pursue promising research directions due to business model considerations of profit potential and investment risk. (being patient)
“Generation” study is testing drug to prevent Alzheimers
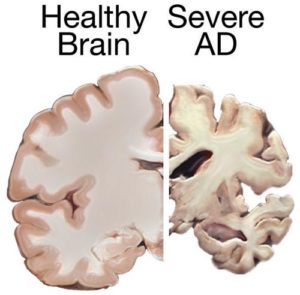 For the first time, people at risk of Alzheimer’s will have access to a new-generation drug to prevent the disease from occurring or slow it down. The “Generation” Study is recruiting thousands of people with copies of the APOE4 gene associated with Alzheimer’s for a trial of a new drug therapy. The drug is designed to reduce the activity of the BACE enzyme and reduce the further accumulation of beta amyloid in the brain. (EXPRESS)
For the first time, people at risk of Alzheimer’s will have access to a new-generation drug to prevent the disease from occurring or slow it down. The “Generation” Study is recruiting thousands of people with copies of the APOE4 gene associated with Alzheimer’s for a trial of a new drug therapy. The drug is designed to reduce the activity of the BACE enzyme and reduce the further accumulation of beta amyloid in the brain. (EXPRESS)
Parkinson’s clinical trials need early stage patients
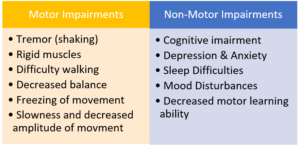 A Florida scientist says sponsors and care providers need to recruit Parkinson’s sufferers as soon as they are diagnosed with mild motor features, before they require meds. Recent discoveries have given researchers hope that they are on a path toward effective treatments, but that won’t happen unless we have enough patients for clinical trials at the earliest stages of the disease. Currently, less than 3 percent of Parkinson’s patients enroll in clinical trials. (CenterWatch)
A Florida scientist says sponsors and care providers need to recruit Parkinson’s sufferers as soon as they are diagnosed with mild motor features, before they require meds. Recent discoveries have given researchers hope that they are on a path toward effective treatments, but that won’t happen unless we have enough patients for clinical trials at the earliest stages of the disease. Currently, less than 3 percent of Parkinson’s patients enroll in clinical trials. (CenterWatch)
