Matthew Geriak, Pharm.D. is an Investigational Research Pharmacist at an institution in San Diego, California, and an Adjunct Clinical Associate Professor at Touro University California. He has 22 years of experience in hospital pharmacy and 10 years’ experience as an Investigational Research Pharmacist. He received his Pharm.D. from the University Read more
Use of surrogate end points shortens cancer trials
The use of surrogate end points (progression-free survival) (PFS) in oncology trials reduced the time needed to conduct clinical trials by 11 months compared to the study duration required to assess the overall survival (OS) benefit of a cancer drug. (MedicalResearch.com)
Profit motive and aversion to risk limit Alzheimer’s progress
Large pharmaceutical companies have been cutting back on their in-house Alzheimer’s research and development. The lack of progress in the fight against Alzheimer’s likely has more to do with the world of finance than the world of biology. Pharmaceutical companies are not able to pursue promising research directions due to business model considerations of profit potential and investment risk. (being patient)
Using sound and light: a new way to perform drug-based studies?
www.scienceandtechnologyresearchnews.com
 An international team has developed an innovative new way to hold samples using sound while they are gently imaged using light. The normal way to immobilize an object would be to use a gel, but introducing drugs to the sample could be slow and unpredictable using this method. Using sound and light is a new way to perform drug-based studies. (Science & Technology Research News)
An international team has developed an innovative new way to hold samples using sound while they are gently imaged using light. The normal way to immobilize an object would be to use a gel, but introducing drugs to the sample could be slow and unpredictable using this method. Using sound and light is a new way to perform drug-based studies. (Science & Technology Research News)
New FDA Guidance. February 2019
 The purpose of this guidance is to assist sponsors of drug and biological products for the treatment or prevention of rare diseases in conducting more efficient and successful drug development programs. Although the statutory requirements for marketing approval for drugs to treat rare and common diseases are the same and issues discussed here are encountered in other drug development programs, these issues are frequently more difficult to address in the context of a rare disease. (fda.gov)
The purpose of this guidance is to assist sponsors of drug and biological products for the treatment or prevention of rare diseases in conducting more efficient and successful drug development programs. Although the statutory requirements for marketing approval for drugs to treat rare and common diseases are the same and issues discussed here are encountered in other drug development programs, these issues are frequently more difficult to address in the context of a rare disease. (fda.gov)
Freelance writer positions available
“Clinical Research Currents” is looking for individuals who would like to write clinical research-related articles for our newsletter and website. This could involve: identify the topic, conduct interviews, write up, and publish. We can help you by offering ideas for articles, or helping with interview questions, as you wish. Contact: Read more
Why do Phase 3 trials fail?
 Drug development is marked by high attrition rates, and the third phase is the most critical. A recent commentary reviewed high profile cases of failed drugs to identify the sources of late-stage failure, and classified the causes of drug failure into avoidable and unavoidable errors. Avoidable errors arise from a lack of scientific rigour, while unavoidable errors arise due to a deficiency in scientific knowledge. (Medical News Bulletin)
Drug development is marked by high attrition rates, and the third phase is the most critical. A recent commentary reviewed high profile cases of failed drugs to identify the sources of late-stage failure, and classified the causes of drug failure into avoidable and unavoidable errors. Avoidable errors arise from a lack of scientific rigour, while unavoidable errors arise due to a deficiency in scientific knowledge. (Medical News Bulletin)
Could we treat age-related diseases with “senolytics”?
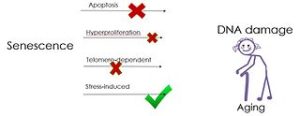 University of Texas Health San Antonio researchers became the first to publish results of the treatment of an age-related disease, idiopathic pulmonary fibrosis (IPF), in human patients with drugs called senolytics. Previously, no published data demonstrated that drugs targeting cellular senescence could be safely given to adults or that they might be used to treat diseases of aging. (ScienceDaily)
University of Texas Health San Antonio researchers became the first to publish results of the treatment of an age-related disease, idiopathic pulmonary fibrosis (IPF), in human patients with drugs called senolytics. Previously, no published data demonstrated that drugs targeting cellular senescence could be safely given to adults or that they might be used to treat diseases of aging. (ScienceDaily)
Infographic: Women in clinical research through the years
Women have seen deficits and gains in their attempts to be included in clinical research, but as 2019 approaches equality seems forthcoming. (Outsourcing-Pharma.com)
Nearly 300 cell and gene therapies are in development
 The new field of cell and gene therapy is part of a new era of medicine, regenerative medicine, which is transforming treatment options for some patients. Today, there are nearly 300 novel cell and gene therapies in development for a range of diseases and conditions. (Cision PR Newswire)
The new field of cell and gene therapy is part of a new era of medicine, regenerative medicine, which is transforming treatment options for some patients. Today, there are nearly 300 novel cell and gene therapies in development for a range of diseases and conditions. (Cision PR Newswire)
- 1
- 2
- 3
- …
- 9
- Next Page »
