 An international team has developed an innovative new way to hold samples using sound while they are gently imaged using light. The normal way to immobilize an object would be to use a gel, but introducing drugs to the sample could be slow and unpredictable using this method. Using sound and light is a new way to perform drug-based studies. (Science & Technology Research News)
An international team has developed an innovative new way to hold samples using sound while they are gently imaged using light. The normal way to immobilize an object would be to use a gel, but introducing drugs to the sample could be slow and unpredictable using this method. Using sound and light is a new way to perform drug-based studies. (Science & Technology Research News)
Using sound and light: a new way to perform drug-based studies?
www.scienceandtechnologyresearchnews.com

 The FDA defines gene therapy as a medical technique that works to modify a person’s genes to treat or cure disease. Gene therapy represents a paradigm shift in the U.S. healthcare from chronic, often lifelong treatments to the potential for cure. There are currently 3 FDA-approved gene therapy products on the market, and nearly 2,600 gene therapy clinical trials are in progress or have been completed. (Pharmacy Times)
The FDA defines gene therapy as a medical technique that works to modify a person’s genes to treat or cure disease. Gene therapy represents a paradigm shift in the U.S. healthcare from chronic, often lifelong treatments to the potential for cure. There are currently 3 FDA-approved gene therapy products on the market, and nearly 2,600 gene therapy clinical trials are in progress or have been completed. (Pharmacy Times)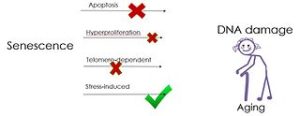 University of Texas Health San Antonio researchers became the first to publish results of the treatment of an age-related disease, idiopathic pulmonary fibrosis (IPF), in human patients with drugs called senolytics. Previously, no published data demonstrated that drugs targeting cellular senescence could be safely given to adults or that they might be used to treat diseases of aging. (ScienceDaily)
University of Texas Health San Antonio researchers became the first to publish results of the treatment of an age-related disease, idiopathic pulmonary fibrosis (IPF), in human patients with drugs called senolytics. Previously, no published data demonstrated that drugs targeting cellular senescence could be safely given to adults or that they might be used to treat diseases of aging. (ScienceDaily)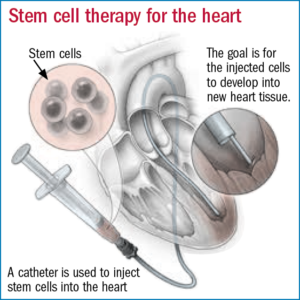 The National Heart, Lung, and Blood Institute (NHLBI) has paused a clinical trial testing an experimental stem cell therapy in heart failure patients. NHLBI paused the study because of recommendations to retract 31 journal articles from the lab of a controversial cardiac stem cell researcher in related fields of cell therapy research, which raised concerns about the scientific foundations of this trial. (STAT)
The National Heart, Lung, and Blood Institute (NHLBI) has paused a clinical trial testing an experimental stem cell therapy in heart failure patients. NHLBI paused the study because of recommendations to retract 31 journal articles from the lab of a controversial cardiac stem cell researcher in related fields of cell therapy research, which raised concerns about the scientific foundations of this trial. (STAT) Virtual clinical trials eliminate the need for study sites. Despite their benefits, fully-virtual trials remain an exception. The hybrid model which allows sponsors to perform some procedures at sites but still reduces the number of sites has come to be popular. Regional variation in laws and regulations is one of the few impediments to hybrid and virtual trials. (outsourcing-pharma.com)
Virtual clinical trials eliminate the need for study sites. Despite their benefits, fully-virtual trials remain an exception. The hybrid model which allows sponsors to perform some procedures at sites but still reduces the number of sites has come to be popular. Regional variation in laws and regulations is one of the few impediments to hybrid and virtual trials. (outsourcing-pharma.com)