 Cell and gene therapies are entering the clinical space. There are currently more than 2,900 registered clinical trials in gene therapy, and nearly 1,000 more gene therapy clinical trials currently enrolling human research participants. There are almost 300 ongoing Phase III trials in gene therapy. Genetic engineering is also enabling growth of the pharmaceutical therapy. (BioPharma-reporter.com)
Cell and gene therapies are entering the clinical space. There are currently more than 2,900 registered clinical trials in gene therapy, and nearly 1,000 more gene therapy clinical trials currently enrolling human research participants. There are almost 300 ongoing Phase III trials in gene therapy. Genetic engineering is also enabling growth of the pharmaceutical therapy. (BioPharma-reporter.com)
Focus of genomic-driven clinical trials shifts to rare cancers
There is a vibrant field of clinical research into new approaches for tackling cancer, and the picture is particularly positive for patients with rare tumor types that have received limited attention in the past. As a proportion of the patients in phase 1 studies, the common cancer types are decreasing and the uncommon types are increasing. (Medical Xpress)
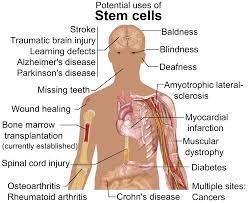
Target cancer stem cells and revolutionize cancer treatment
About 95-98% of new anti-cancer drugs fail phase III clinical trials. Most drugs are made to target “bulk” cancer cells, but not the root cause: the cancer stem cell. New therapies that specifically target and eradicate these cancer stem cells are needed to prevent tumors from growing and spreading, and we may then be able to turn cancer into a manageable chronic disease. (INDEPENDENT)
FDA guidance promises flexibility for stem cell trials
In a final guidance issued last week, “Expedited Programs for Regenerative Medicine Therapies for Serious Conditions”, regulators are promising flexibility in clinical trial design for stem cell therapies in rare diseases. The FDA is also promising to be flexible about fast-tracking some potential stem cell treatments. (CenterWatch)
A primer on gene therapy
 The FDA defines gene therapy as a medical technique that works to modify a person’s genes to treat or cure disease. Gene therapy represents a paradigm shift in the U.S. healthcare from chronic, often lifelong treatments to the potential for cure. There are currently 3 FDA-approved gene therapy products on the market, and nearly 2,600 gene therapy clinical trials are in progress or have been completed. (Pharmacy Times)
The FDA defines gene therapy as a medical technique that works to modify a person’s genes to treat or cure disease. Gene therapy represents a paradigm shift in the U.S. healthcare from chronic, often lifelong treatments to the potential for cure. There are currently 3 FDA-approved gene therapy products on the market, and nearly 2,600 gene therapy clinical trials are in progress or have been completed. (Pharmacy Times)
Sloppy followup of Chinese gene-editing experiments worries Crispr-Cas9 inventors
 Chinese scientists have been forging ahead in experimenting with gene-editing on humans in the last few years, using a powerful tool called Crispr-Cas9 to edit the DNA of dozens of cancer patients. However, no federal body is overseeing these trials in China, and at least one such trial has lost touch with patients whose DNA was altered. Long-term followup of these patients is essential because unintended consequences of gene therapy could surface years later. (The Wall Street Journal)
Chinese scientists have been forging ahead in experimenting with gene-editing on humans in the last few years, using a powerful tool called Crispr-Cas9 to edit the DNA of dozens of cancer patients. However, no federal body is overseeing these trials in China, and at least one such trial has lost touch with patients whose DNA was altered. Long-term followup of these patients is essential because unintended consequences of gene therapy could surface years later. (The Wall Street Journal)
Nearly 300 cell and gene therapies are in development
 The new field of cell and gene therapy is part of a new era of medicine, regenerative medicine, which is transforming treatment options for some patients. Today, there are nearly 300 novel cell and gene therapies in development for a range of diseases and conditions. (Cision PR Newswire)
The new field of cell and gene therapy is part of a new era of medicine, regenerative medicine, which is transforming treatment options for some patients. Today, there are nearly 300 novel cell and gene therapies in development for a range of diseases and conditions. (Cision PR Newswire)
Growing cancer drugs in chicken eggs could lower the cost of drugs by 90%
Japanese researchers may have found a way to produce cheaper drugs from chicken eggs. They have successfully genetically modified hens using CRISPR to produce eggs containing large amounts of interferon beta protein, a very expensive protein used to treat various illnesses including multiple sclerosis and cancer. The protein costs between $300-$1000 for just one microgram procured with current methods; for treating MS, the interferon dosage can start at 30 micrograms. This technology could reduce the price of cancer drugs by 90% if proven successful in further trials. (CNN)
The present and future of genetic counseling: an interview with Emily Quinn, a certified genetic counselor. Part 3
Interview and article by Tatsiana “Tanya” Verstak* Part 3: Utilizing genetic information in clinical care Tanya Verstak For the most part, are people trusting genetic information and do they want to have this testing done? Emily Quinn I think so. Probably the big concern in our field is that I Read more
CRISPR- Cas9 finalist for “best technology” in Tech Crunch’s Crunchies Awards
FEBRUARY 6, 2017 | SAN FRANCISCO WAR MEMORIAL OPERA HOUSE
THE OSCARS OF STARTUPS AND TECHNOLOGY
TechCrunch kicks off 2016 with the 10th Annual Crunchies Awards Show, the award ceremony to recognize and celebrate the most compelling startups, internet, and technology innovations of the year.
10th Annual Crunchies Finalists in the category of Best Technology
An exceptional recent technological achievement
Blue Origin
CRISPR- Cas9
Facebook solar plane
SpaceX Falcon 9 Landing
Tesla Solar Roof
WTF is CRISPR?
Say you’ve inherited a rare genetic mutation that guarantees you’ll get a certain form of cancer by the time you reach 50 years of age. And that this is most likely how you are going to die. But what if I told you this cancer gene, passed down from generation to generation, can be snipped out of your genome entirely and you never pass it on to any of your offspring?
That is the promise of CRISPR, which not only has the potential to radically change the genetic code of all of humanity but could also fundamentally affect our health care, food system, farming and even the manufacturing and production industries.
In short, it’s the technological discovery of the century. CRISPR (pronounced crisper) stands for clustered regularly interspaced short palindromic repeats. And it’s a sort of immunity system set up in the genetic code of our cells. (tech crunch.com)
