 The U.S. Food and Drug Administration (FDA) proposed changes that would allow institutional review boards (IRBs) to waive or alter requirements for obtaining informed consent for clinical trials involving minimal risk to participants. Under current FDA regulations, exceptions for obtaining informed consent can only be made in life-threatening situations or when conditions for emergency research are met. (Regulatory Focus)
The U.S. Food and Drug Administration (FDA) proposed changes that would allow institutional review boards (IRBs) to waive or alter requirements for obtaining informed consent for clinical trials involving minimal risk to participants. Under current FDA regulations, exceptions for obtaining informed consent can only be made in life-threatening situations or when conditions for emergency research are met. (Regulatory Focus)
“Vague” terms downplay the harms of cancer drugs
 A recent study published in the British Medical Journal found that results of oncology clinical trials published in major medical journals were described using vague terms that downplayed harms associated with cancer drugs, which was at odds with side effects observed in the actual data. Journalists also use these “vague terms” that minimize potential harms, while emphasizing and exaggerating the potential benefits of new health care interventions. (healthnewsreview.org)
A recent study published in the British Medical Journal found that results of oncology clinical trials published in major medical journals were described using vague terms that downplayed harms associated with cancer drugs, which was at odds with side effects observed in the actual data. Journalists also use these “vague terms” that minimize potential harms, while emphasizing and exaggerating the potential benefits of new health care interventions. (healthnewsreview.org)
National institute pauses heart failure study after papers retracted
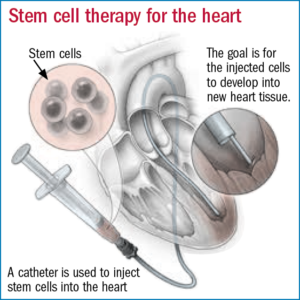 The National Heart, Lung, and Blood Institute (NHLBI) has paused a clinical trial testing an experimental stem cell therapy in heart failure patients. NHLBI paused the study because of recommendations to retract 31 journal articles from the lab of a controversial cardiac stem cell researcher in related fields of cell therapy research, which raised concerns about the scientific foundations of this trial. (STAT)
The National Heart, Lung, and Blood Institute (NHLBI) has paused a clinical trial testing an experimental stem cell therapy in heart failure patients. NHLBI paused the study because of recommendations to retract 31 journal articles from the lab of a controversial cardiac stem cell researcher in related fields of cell therapy research, which raised concerns about the scientific foundations of this trial. (STAT)
Minnesota hospital doses emergency patients without informed consent
 The U.S. Food and Drug Administration (FDA) has cited the Hennepin County Medical Center in Minnesota over its institutional review board’s (IRB) oversight of clinical trials including one case involving dosing emergency patients with ketamine without the patients’ consent. Public Citizen called on the FDA to suspend the hospital’s IRB until they took corrective steps to ensure adequate protections for research subjects going forward. (Regulatory Focus)
The U.S. Food and Drug Administration (FDA) has cited the Hennepin County Medical Center in Minnesota over its institutional review board’s (IRB) oversight of clinical trials including one case involving dosing emergency patients with ketamine without the patients’ consent. Public Citizen called on the FDA to suspend the hospital’s IRB until they took corrective steps to ensure adequate protections for research subjects going forward. (Regulatory Focus)
Off-label use of drugs based on flimsy evidence
 Clinical trials that explore the repurposing of drugs for off-label uses are common, but without a commitment to rigorously testing the hypotheses generated by these exploratory trials, drugs used off-label are costly and may be ineffective. Ethics committees should devise measures that encourage coupling of exploratory testing with confirmatory trials. (British Medical Journal)
Clinical trials that explore the repurposing of drugs for off-label uses are common, but without a commitment to rigorously testing the hypotheses generated by these exploratory trials, drugs used off-label are costly and may be ineffective. Ethics committees should devise measures that encourage coupling of exploratory testing with confirmatory trials. (British Medical Journal)
Phase 1 pediatric cancer trials are therapeutic. True? Or, is this “a noble lie”?
 It’s this author’s view that it may be a mistake to think of early phase trials as having a therapeutic impetus. U.S. regulations require that, when we enroll children, we view phase 1 trial participation as therapeutic. However, a recent meta-analysis concluded that phase 1 trials in children cannot generally be presented as having a therapeutic risk/benefit. Thus, phase 1 trials in children cannot be reconciled with research regulations. (translationalethics.com)
It’s this author’s view that it may be a mistake to think of early phase trials as having a therapeutic impetus. U.S. regulations require that, when we enroll children, we view phase 1 trial participation as therapeutic. However, a recent meta-analysis concluded that phase 1 trials in children cannot generally be presented as having a therapeutic risk/benefit. Thus, phase 1 trials in children cannot be reconciled with research regulations. (translationalethics.com)
Doctor protests use of placebos on children in asthma study
 A study that explores whether vitamin D prevents asthma attacks in children is being criticized as unethical. All of the study participants have serious asthma and low levels of vitamin D, but only half of the participants in the trial are getting high doses of vitamin D, while the other half will receive a placebo, leaving their clinically low level of vitamin D untreated. (Boston Globe)
A study that explores whether vitamin D prevents asthma attacks in children is being criticized as unethical. All of the study participants have serious asthma and low levels of vitamin D, but only half of the participants in the trial are getting high doses of vitamin D, while the other half will receive a placebo, leaving their clinically low level of vitamin D untreated. (Boston Globe)
